Migration Information and FAQs
Information You Need to Review for Your Studies That Have Migrated
Please refer to this document when you submit your first action to the IRB for a migrated study. Please refer to this document for additional information about process changes now in PROTECT.
To complete your first scientific review for a migrated protocol, please refer to this document.
Note that Exempt protocols and NHSR projects will not migrate. If you need to submit a modification for one of these types of protocols in PROTECT, enter it as a new project but refer back to the previously approved protocol and indicate that you are now submitting a modification.
Information About External (Ceded) Study Update vs Site Mod
Please refer to this document for more information regarding study update vs site MODs.
Frequently Asked Questions
LOGIN/ACCOUNTS/HELP/SUPPORT
A link to our new PROTECT system can be found on our OHRSP website.
Visit our Office of Human Subjects Research Protections website and click the banner that says “Visit the NIH PROTECT Project Page…”. Here you will find links to find PROTECT experts, past trainings or you can submit a help desk ticket.
All recordings of the live user trainings delivered by OHSRP can be found on our SharePoint site. You can login using your NIH credentials.
The PI, the Proxy(ies), and the Primary Contact.
Only users who need to login to PROTECT and access/interact with projects need to have an account. People do not need an account to be added as a study team member to a protocol.
Submit a help desk ticket to request an account. We have a new help desk link now: https://ohsrp.helpdesk.nih.gov
NOTE - the process for requesting an account has not changed.
OHSRP has coordinated with NIH institutes and centers to identify and train representatives from as many ICs as possible to be considered system “experts”. These individuals received system training directly from Huron and are available to be your first line of contact for day-to-day system questions. These experts can be found on our website, here: NIH PROTECT Experts.
Please consult these experts first for your initial questions about the system so you may receive assistance right away. They can assist you in elevating your request to our Help Desk if additional assistance is needed.
All personnel who are engaged in Human Subjects Research (HSR) (e.g. associate investigators) who are working under the supervision of the NIH PI should be listed under the “Local Study Team Members” section of the study application. Contractors and Fellows, etc. (anyone with a NED account) should also be listed here if they are engaged in HSR. These personnel will no longer be separated out in a different section.
Study members who are working under the supervision of the NIH PI and are engaged in HSR but who are not in NED should be listed as an external team member.
“Protocol Navigator” is an administrative role created to allow people in this role to have read/write access to the study and to be an option to be selected as a proxy for the PI. Only people who are serving in a regulatory role for the study who are NOT engaged in human subjects research should be identified in this role. They may be added the same way as a study team member and “Protocol Navigator” is a drop-down choice for any user when you select their name on the form.
Study Contacts in iRIS will be merged over into the “Guest” tab of a study in PROTECT and these users will have read-only access to the project. They will not be able to edit a project or push it through the workflow.
PROXIES
A proxy is a member of the study team listed on the application that the PI designates to act on their behalf. The proxy can do all the same actions in the system that the PI can with the exception of submitting the Scientific Review. The PI MUST submit the Scientific Review in the system.
Anyone listed on the study application as local study team members can be a proxy. These are the only personnel you will be able to choose on the Assign Proxy activity.
Only the PI can assign/remove a proxy.
Yes, a PI can assign any IRB approved local study team member listed on the application to be a proxy.
The PI will log into PROTECT, navigate to the study workspace, and click the “Assign PI Proxy” activity listed on the left side to add or remove proxies.
No, only the PI can assign or remove proxies.
A proxy can initiate and submit all actions in the system except when it comes to Scientific Review. A proxy can initiate and draft the Scientific Review but only the PI can ultimately submit the Scientific Review form.
NOTE - RNIs can be submitted at any time and by any system user.
The Primary Contact receives all study notifications. They have read-only access to the project and only one person can be designated as a Primary Contact.
Only one Primary Contact can be assigned. However, if you have a need to make sure multiple people receive all notifications, you may want to assign them as Proxies or create a group account in Outlook that can be added as a user in PROTECT.
Yes, a group e-mail can be used. To do this, a dummy user account has to be created by us and the general mailbox email address associated with that user account. To request one of these accounts, you would submit a Help Desk ticket.
GUESTS
A guest is someone who has read-only access to the project. A user can be designated as a guest at any time (no modification needed). A guest can be an NIH employee or offsite personnel, but must be a person who has a NED account. There is no limit to the number of guests that can be added.
SUBMITTING
Submit permissions
| Project | Who Can Submit |
|---|---|
| IRB | PI & Proxies |
| IRB RNIs | Any user in the system |
| SRC | Only PI can submit |
| RSC | PI & Proxies |
No. A RNI can be submitted at ANY time by ANY system user. It can be associated with one or more studies, or even no study at all. This is so that all RNIs can be sent to the IRB in as timely a manner as possible.
NOTIFICATIONS
You will still receive CR reminders, but they will come from PROTECT. Your dashboard will also show all of your studies that are expiring soon in the left-hand corner. They are color coded as follows:
| Blue | The study expiration date is between 60 and 15 days away. |
|---|---|
| Orange | The study expiration date is 15 days or less before the expiration date. |
| Red | Today is the expiration date or it is within 6 days after the expiration date. |
| Disappears when | 6 days have passed since the expiration date. |
Yes, we have a list of various reports under the Reports tab under the IRB project. You will only see information in the reports that pertains to studies that you have access to in PROTECT.
No, these studies will not migrate. These will have to be entered as a new study in PROTECT only if you have a need to modify them later. If you need to modify these, be sure to refer to the initial IRB # that was already assigned to your submission in iRIS.
No, it will not. Studies that have an action still in process in iRIS at the time of our first migration (go-live date) will not be available in PROTECT initially. These studies will be migrated to PROTECT in a “second wave” migration about one month after the first go-live migration. After this point, any pending action that is not approved in iRIS that you wish to pursue would need to be re-entered into PROTECT and reference the IRB# from iRIS.
No, this will also have to be entered as an Initial Submission in PROTECT. See instructions here for how to enter the first scientific review for either a modification, annual review, or quadrennial review. If you have initial scientific review approval for a project not yet submitted to the IRB as an initial review, you must initiate scientific review in PROTECT for that new protocol, complete the form, and attach your approval. It will not need to be reviewed again by the Scientific Review Committee (SRC); however, it will be processed through by the SRC coordinator so that the project outcome is recorded in the system.
Documents/Miscellaneous
Yes, iRIS will remain in a read-only format for approximately one year post Go Live. During this year, you can go in and download anything you want to keep from the system before we archive it.
No, study teams should only upload clean Word documents in PROTECT. There is a compare function in PROTECT which will create a tracked Word version that you can download and save if you wish, and that all reviewers can use during their review.
These are located in the top right-hand corner of any project workspace for each submission.
These will be found in your IRB approval letters.
You will see in the History tab of the project that your ancillary reviewer has taken action; or under the Reviews tab next to the reviewer’s name, it will say “Yes” under the “Accepted” heading.
To add a new document in PROTECT, click “add” button in the study application anywhere documents can be uploaded and simply upload the file.
To revise an existing document in PROTECT, click the “update” button in the study application next to the document you have a revision for. You will see a pop-up to upload a revised document. Upload your file and it “stacks” it for you without any cumbersome checking in and out like we did in iRIS. You will then be able to see previous versions of the documents under the “Documents” tab by clicking on “History”.
When you upload a document to PROTECT, it has a field for “Name”. You should be accustomed to naming your files per the naming convention our website that can be found HERE. If you continue to do this, and leave the Name field blank, the system will automatically populate the file name in to the “Name” section so that it is easy to distinguish on the Documents tab later.
OPS/Clinical Trials.gov/PRIA
OPS has developed their own interface called Protocol Query System (PQS) in which research teams can enter their own information for OPS to review, as well as the Planned and Cumulative Enrollment Data. In every project workspace of PROTECT there is a shortcut link which will take you directly to that database.
PQS also provides the ability to search and/or update protocol data not collected in PROTECT, and pulls data PROTECT collects for IRB submissions. Investigators/protocol navigators/study team can update the protocol information using the different action types. OPS will continue to receive protocol actions from PROTECT, once approved. For new protocols, a “PROTECT Protocol Information” action can be created in PQS as the protocol undergoes IRB review. It’s important that upon notification of approval by the IRB that the “PROTECT Protocol Information” action be submitted immediately to marry the data. In the absence of a submitted “PROTECT Protocol Information” action, OPS will activate the protocol using the PI Inst/Branch as the funding institute/branch and the accrual status set to “Not Yet Recruiting.” In addition, the study team will be notified of the urgency to complete the “PROTECT Protocol Information” action, to avoid delaying registration on clinicaltrials.gov. Questions related to the process or requirements can be sent to CC_Protocol_Serves@cc.nih.gov
The PRIA is a prospective review of protocols to ensure that CC departments, consult services, procedure and testing areas have adequate staffing, resources and/or equipment in order to support the implementation of the protocol. The PRIA process is a targeted comprehensive review of the protocol and resource needs conducted in collaboration with the Principal Investigator (PI), key contacts in CC departments, testing and procedure areas, and IC consult services. The PRIA process is separate and independent of the IRB review process. Protocols that receive IRB approval cannot enroll participants until the PRIA is approved; this ensures that there are adequate resources to support the protocol implementation. The PRIA is submitted by PI/Research Teams at the time of scientific review of the protocol. For questions about the PRIA review process email: PRIASubmission@mail.nih.gov.
Multi-site
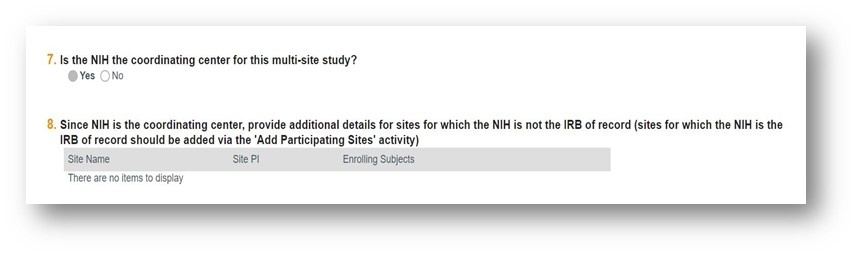
How to document situations where NIH is the coordinating center in PROTECT
When NIH is the coordinating center in a multi-site study the response to Q. 7 in the Basic Study Information section should be marked “Yes”. The PROTECT form will branch to Q.8 (below) which requests that all participating sites that are not relying on the NIH IRB be listed. This means that all sites that are engaged in human subjects research (HSR) (i.e., enrolling subjects, working with identifiable data/specimens, working with coded data/ samples and have access to the code key) and that are using their local IRB or ethics board to provide oversight should be listed. This includes international sites with whom NIH is working. Collaborators at other sites that are not engaged in HSR should not be listed e.g., those only working with coded data/specimens with no access to a code key or working only with deidentified data/ specimens.
Excerpt from Basic Study Information section
For each site, please provide the Site Name, the Site PI, and identify whether they are currently enrolling by marking “Yes” or “No” in that column.
- The response should be marked “Yes” when a site is engaged in HSR and is actively enrolling subjects.
2. The response should be marked “No” when:
- A site is engaged in HSR but does not plan to ever enroll subjects and is only working with data/specimens, or
- A site was enrolling subjects but is no longer enrolling and plans to only work with identifiable data/specimens or coded data/specimens and has access to the code key, or
- A site was enrolling subjects and has now closed, or
- A site was only working with data/specimens and has now closed.
At the time of CR, NIH study teams with sites that fall into categories 2.a or 2.b must provide confirmation that there continues to be local IRB approval for those sites or a closure letter if the site has closed.
NOTE: Any site that is engaged in HSR and is relying on the NIH IRB should not be identified in Q. 8.